
Problem #9: The density of an aqueous solution of nitric acid is 1.430 g/mL. Use MV = grams / molar mass to determine the volume.

Problem #8: What is the density (in g/mL) of a 3.60 M aqueous sulfuric acid solution that is 29.0% H 2SO 4 by mass?ġ) Assume 100.0 g of solution is present.Ģ) Detemine the volume of solution that weighs 100.0 g:

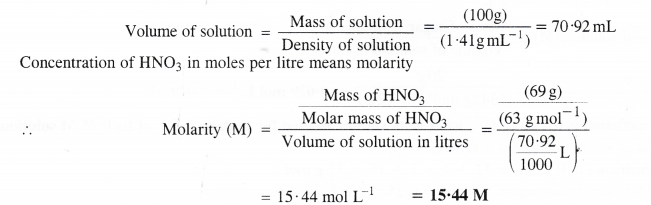
Compute the density of this solution.Ģ) Compute the mass of the above solution:ġ000 g solvent + (8.9660 moles x 60.05 g/mol) = 1538.41 gģ) Use molarity to get the volume of solution containing 8.9660 moles Problem #7: An aqueous acetic acid solution is simultaneously 6.0835 molar and 8.9660 molal. A solution is made by dissolving 9.660 g of thiophene in 260.0 mL of toluene.Ī) Calculate the molality of thiophene in the solution.ī) Assuming that the volumes of the solute and solvent are additive, determine the molarity of thiophene in the solution. Problem #6: The density of toluene (C 7H 8) is 0.867 g/mL, and the density of Special bonus part to Problem #5: In the above solution, what is the mole fraction of HNO 3?Ģ) Determine the moles of each substance: What is the molarity and the molality of the acid?ġ) Determine moles of HNO 3 in 100.0 g of 70.4% solution:ġ.11725 mol / 0.070422535 L = 15.86 M = 15.9 M (to three sf) Problem #5: Concentrated nitric acid is a solution that is 70.4% HNO 3 by mass. What are the molality and molarity of HF in this solution? Problem #4: An aqueous solution of hydrofluoric acid is 30.0% HF, by mass, and has a density of 1.101 g cm -3. How do I figure out the molarity?ġ) Assume 100.0 g of solution is present. Its strength is 32.0% and its density is 0.89 g/mL.

Use density to get mass:Ģ) (a) Use percent mass to get mass of HCl, then (b) convert to moles:
What is its molar concentration? (The density of the solution is 1.19 g/mL.)ġ) Determine moles of HCl in 100.0 g of 37.7% solution:Ģ) Determine volume of 100.0 g of solution:ġ) Assume 1.00 L of solution. Problem #2: Concentrated hydrochloric acid is usually available at a concentration of 37.7% by mass. Moles H 3PO 4 = 87.0 g / 97.9937 g/mol = 0.8878 molĢ) Calculate the volume of 13.0 M solution which contains 0.8878 mol of H 3PO 4:ģ) Determine the density of the solution:ġ00.0 g / 68.3 mL = 1.464 g/mL = 1.46 g/mL (to three sig fig)ġ) Let us assume 100.0 grams of solution. If it is 13.0 M, what is the density of this solution? What is its molality?ġ) Determine the moles of H 3PO 4 in 100.0 grams of 87.0% solution: Problem #1: Phosphoric acid is usually obtained as an 87.0% phosphoric acid solution. ChemTeam: Calculations involving molality, molarity, density, mass percent, mole fraction (Problems #1 - 10) Calculations involving molality, molarity, density, mass percent, mole fraction


 0 kommentar(er)
0 kommentar(er)
